It has been national bestseller for more than 65 years. What is the difference between molality and molarity.

Difference Between Molarity And Molality Science Hindi Quikr Exam Youtube
In the center column state whether the material is a.

. Several chapters are devoted to developments in 20th century physics especially quantum mechanics and and the extent to which they explain the periodic table in a more fundamental way. Email protecteds1 Rate Law. It really has been that long.
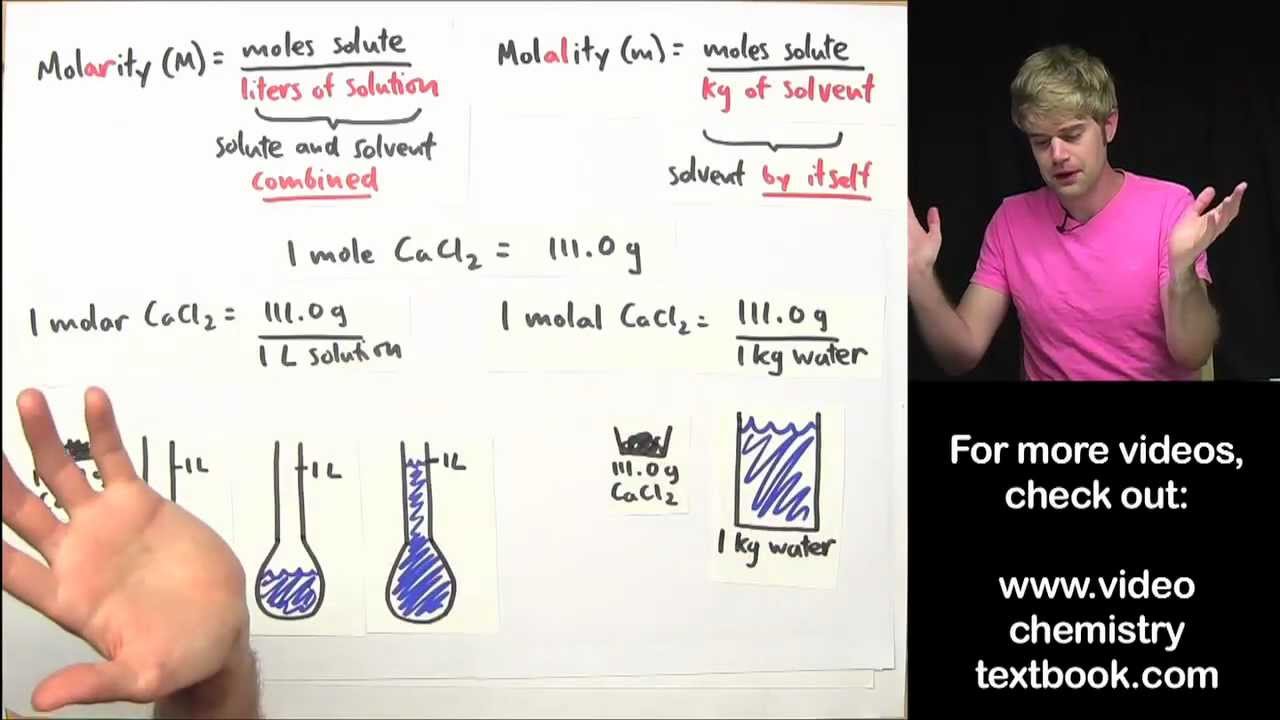
Substances with zero or low electronegativity difference such as H 2 O 2 N 2 CH 4. Preface The Essentials of Physical Chemistry has been written for BSc students. Molality is the number of moles of solute present in 1kg of the solvent.
Worksheet 16 Calorimetry Calorimetry is the experimental measurement of. Molecular mass of Ca 3 PO 4 340231816. Handbook of Chemistry and Physics 84th - David R Lide.
Recall that the electronegativity difference can be used to determine the polarity of a substance. There are many examples in nature and. Calculate the mass percent of calcium phosphorus and oxygen in calcium.
Molarity is the number of moles of solute dissolved in 1 litre of the solution. 1 - Changing the Living World - Answer Key IncludedThis guided reading and review worksheet allows students to go over the section they just learned and answer questions right from the reading. It has been used by more than 2 million students.
Integrated Rate Law Part I Part II. 6 x 10-19 C. Note that full ionic character is rarely reached however when metals and nonmetals form bonds they are named using the rules for ionic bonding.
Examples of Directional Selection Directional selection is fairly easy to recognize because it leads to dramatic shifts toward extreme phenotypes over time. Faradays Law 255. It is 26 editions old.
By taking the difference between the electronegativity values for each of the atoms involved in the bond the bond type and polarity can be predicted. The First Year students of the 2020-21 academic year will take the Higher Secondary examination on 24th of September 2021 and will end on 18th of October. Typically an ionic bond has an electronegativity difference of 18 or above whereas a polar covalent bond is between 04 to 18 and a nonpolar covalent bond is 04 or below.
2H 2 O was made up to 250. Phosphate Ca 3 PO 4 Solution. Figure 86 Electronegativity Difference Diagram.
0 Comments